Hot and sour: parasite adaptations to honey bee body temperature and pH, and what it could mean for beekeeping and neglected tropical diseases
Nov 26, 2021
This post references our new article in Proceedings of the Royal Society B: Hot and sour: parasite adaptations to honey bee body temperature and pH (https://doi.org/10.1098/rspb.2021.1517).
You can find the preprint on Biorxiv (https://doi.org/10.1101/2021.07.03.447385) and a video summary of our findings on Youtube (https://youtu.be/77zNO8vCSSg).
Please cite this work as:
Palmer-Young EC, Raffel TR, Evans JD. 2021 Hot and sour: parasite adaptations to honeybee body temperature and pH. Proc. R. Soc. B 288: 20211517. https://doi.org/10.1098/rspb.2021.1517
What’s cooking in the bee gut?
After eight years studying the bumble bee parasite Crithidia bombi, I’ve recently turned my attention to the related honey bee parasites Crithidia mellificae and Lotmaria passim, the latter first described at my new academic home in the USDA Bee Research Laboratory [1]. These parasites are in the trypanosomatid family– phylogenetic relatives of the insect-vectored neglected tropical diseases African sleeping sickness, Chagas disease, and leishmaniasis. Although their Latin names are not yet rolling off the tongues of commercial beekeepers, take note that these parasites have been found in over 80% of colonies in some national surveys [2–5], and associated with colony collapse on three continents [2,6,7]. Related parasites in bumble bees have a range of chronic effects, including slowed colony growth, impaired foraging efficiency, and reduced overwinter survival [8–11].
Hot and sour moats against infection?
How could honey bees defend themselves against these emerging infectious diseases? Based on study of other honey ailments, my own work with Crithidia bombi, and the distribution of trypanosomatids in mammals, a few unique aspects of the honey bees’ social lifestyle could provide the upper tarsus. First, the large colonies of honey bees enable them to keep the colony at high temperatures similar to those of warm-blooded mammals. This temperature regulation is quite rare among insects, which are generally thought to be 'cold-blooded’. We can think of colony-level maintenance of high temperatures as a kind of 'social fever' that slows or prevents growth of opportunistic pathogens, and can help to mitigate infections with chalkbrood, DWV, and Nosema [12–14]. Honey bees also have acidic guts [15] as a result of fermentation of their fiber-rich diets by gut bacteria– these pickled intestines can likewise restrict the growth of pathogens, just as they preserve fermented foods like sauerkraut, kimchi, and yogurt.
My previous research showed that both high temperature and acidity—which are considered barriers to the establishment of most insect-borne trypanosomatids in warm-blooded mammals [16]—reduced growth of the related parasite of bumble bees, Crithidia bombi [17,18]. Inoculations of live bees showed that infection was reduced by 80% when bees were reared at 37 °C compared to cooler temperatures [19]. These findings with parasites of bumble bees suggested that high temperatures and gut acidity could also protect against trypanosomatid infection honey bees. On the other hand, these characteristics of honey bees could impose selection on bee parasites that is reflected in higher tolerance of heat and acidity than what’s found in parasites of other, cold-blooded insects.
To address the effects of hive-like temperatures and gut-like acidity levels on parasite growth—and to see whether honey bee parasites display any special adaptations to the high temperatures and low pH levels that they encounter in bees—we compared the temperature and pH tolerance of honey bee parasites with that of related parasites from mosquitoes– which do not inhabit heated colonies and have alkaline rather than acidic guts [20]. If the high temperatures of bee colonies and acidic environment of the bee gut shape the gut parasite community, we would expect a decrease in growth rate over the temperature and pH range found in the colony, and better tolerance of heat and acidity among parasites of honey bees than among parasites of mosquitoes.
Some like it hot
Our first big finding was with the honey bee parasite Crithidia mellificae—described in collapsing honey bee colonies in Australia over 50 years ago [21,22]. This species had greater heat tolerance than any insect-specific trypanosomatid studied to date—suggesting that selection for heat tolerance in the steamy bee colony has been a big factor in the parasite's evolution. Both bee parasites were more heat-tolerant than the parasites of mosquitoes, again indicating that the high temperatures of the bee colony could provide protection against infection by non-specialist parasites (Figure 1).
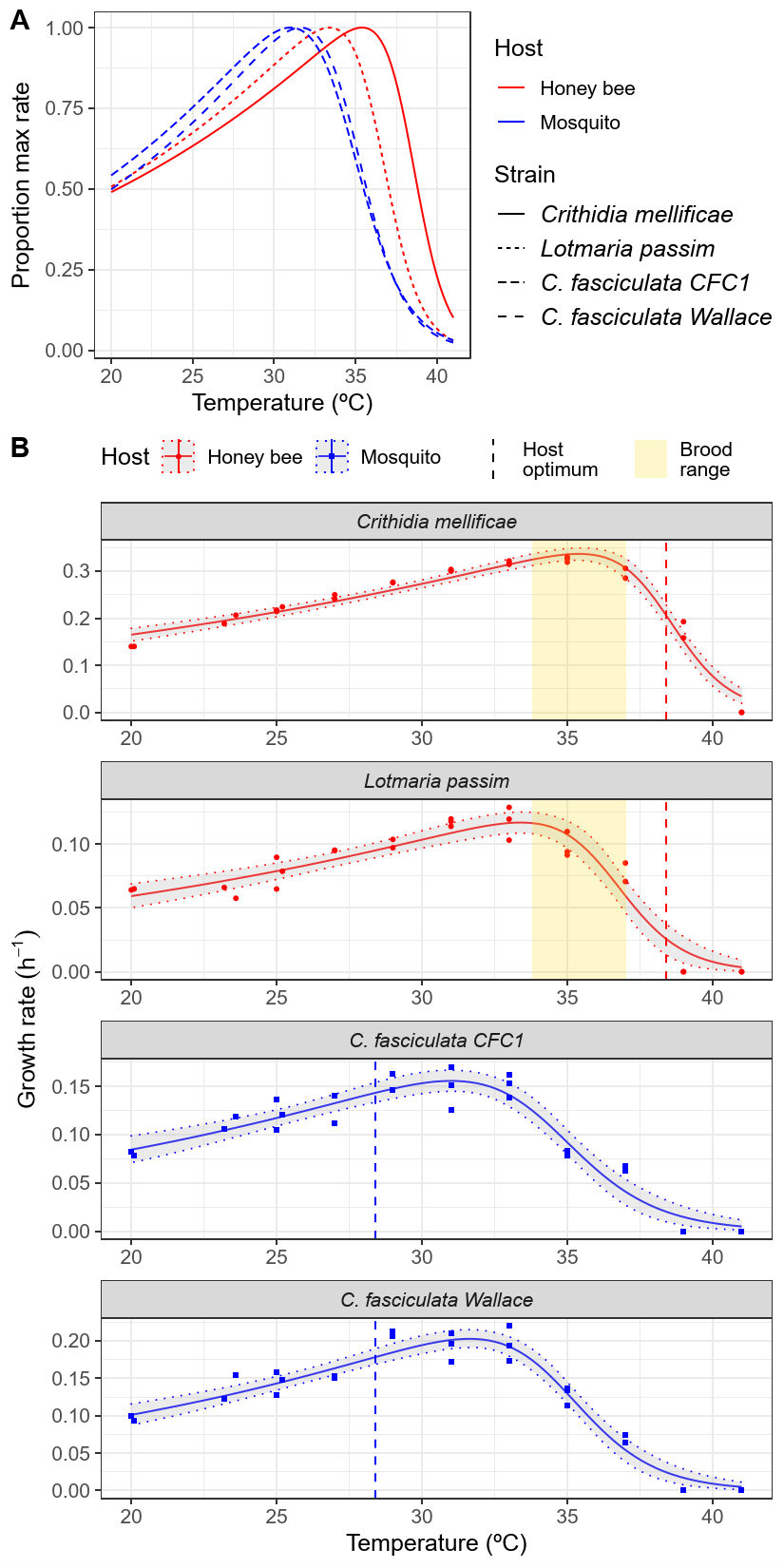
Figure 1: Honey bee parasites had greater heat tolerance than did a parasite from mosquitoes; growth of the emerging parasite Lotmaria passim was inhibited by temperatures found in brood-rearing colonies.
However, the emerging bee parasite Lotmaria passim was significantly less heat-tolerant than was Crithidia mellificae, suffering a 50% reduction in growth rate over the temperature range found in brood-rearing honey bee colonies (Figure 1). This suggests that turning up the heat in the hive—either by changing hive construction, feeding thermogenic foods, or breeding for bees that regulate a high temperature-- could reduce infection of colonies. Conversely, factors that reduce colony temperatures—such as nutritional or chemical adversity in suboptimal landscapes [23]—could make bees more susceptible to infections and their consequences. Although speculative, the relatively low heat tolerance of the emerging Lotmaria raises questions about whether this parasite might have recently jumped from a different bee species with lower brood-rearing temperatures—perhaps spreading from a host with open nest construction and lower temperature set points [24] into cavity-dwelling Apis mellifera.
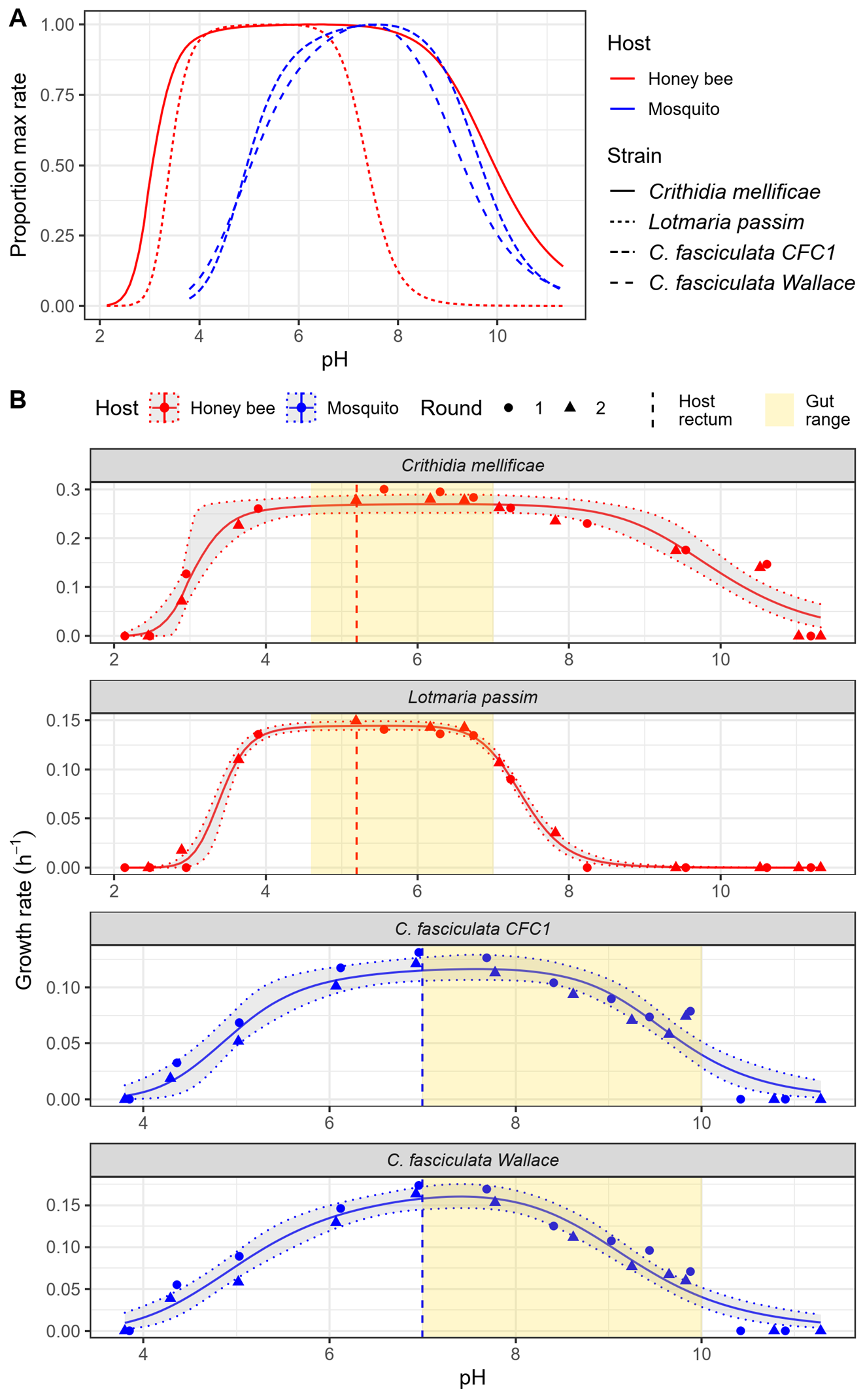
Figure 2. Honey bee parasites were tolerant of acidic conditions found in bee intestines, whereas mosquito parasites were tolerant of alkaline conditions found in mosquito guts. Note the broad pH range of Crithidia mellificae, which has been found in several bee species besides honey bees.
We found that pH tolerance also varied substantially between the bee and mosquito parasites (Figure 2), reflecting differences between the acidic guts of bees and the alkaline guts of mosquitoes– where gut pH can exceed pH 10 in larvae– similar to household ammonia! The honey bee parasites grew at acidity levels a full two pH units lower than the mosquito parasites did, suggesting that the acidic guts of honey bees could limit the types of parasites that can establish in the gut, and indicating a potential role for acid tolerance in host-parasite specificity in insects. The established parasite Crithidia mellificae had a remarkably broad pH range-- able to grow at any pH between 3 and 10 (from vinegar to ammonia!). This could explain why it's been found in a broad range of bee hosts with presumably diverse gut chemistry [1,25]. The emerging parasite Lotmaria appears well-suited to the acidic pH of the bee intestine, but growth was essentially limited to acidic conditions—potentially explaining why this parasite has been found in acid-gutted Apis almost exclusively [1]. Both species had much better acid tolerance than the bumble bee parasites I tested previously [18], suggesting that acid-producing microbiota might not be as effective against infection in honey bees as they are in bumble bees.
Can you make toast with this?
These experiments could be informative for bee management, conservation, and ecology in the face of these emerging infections. For example, they could guide methods of hive construction and management to optimize thermoregulation of colonies; development of diets or probiotics that enhance thermogenesis, and understanding of the seasons when colonies need them most; and if– as some studies suggest– temperature set point is a heritable trait [26], breeding for bees that keep the colony at parasite-inhibiting temperatures. In terms of conservation, these experiments may also predict susceptibility to parasites in hosts that lack colonial thermoregulation and social transmission of core gut bacteria, including many species of managed and wild solitary bees that are likewise exposed to trypanosomatids at flowers [27]. More broadly, these experiments use bees–which are facultatively warm-blooded—as models to better understand the value of energetically intensive temperature regulation for stability of the microbiome, gut chemistry, and host immunity to infection.
Beyond the bee community, these experiments could enhance our understanding of parasite adaptations to heat and acidity that enable them to make the jump from insects to mammals [28]. Recall that these parasites of bees are closely related not just to parasites of mosquitoes, but also to the sand fly-vectored Leishmania that infect 1-2 million humans each year, making them one of the world’s formidable neglected tropical diseases [29]. Heat and acidity are two major barriers to trypanosomatid infection in warm-blooded mammals, where body temperatures are much higher than those in ectothermic insects, and the intracellular Leishmania lives stealthily within an acidic vacuole of the white blood cells [16]. Heat tolerance is also a feature that distinguishes the Leishmania that cause unsightly but rarely fatal skin lesions from those that infect the visceral organs—the liver and spleen—causing over 90% mortality [16,30]. The ~4 °C difference in temperature between skin and visceral lesions is very close to the difference in heat tolerance between the mosquito parasites and Crithidia mellificae from honey bees. Bee parasites could serve as models for the development of heat tolerance in generally insect-restricted parasites that might have the potential for spillover into warm-blooded hosts, given the similarity of colony temperatures to body temperatures in humans and other mammals [31].
In fact, new findings from Brazil suggest that study of heat tolerance in these supposed “insect parasites” may have more applied value than I originally thought. Crithidia mellificae was recently found in diverse wild mammals near Rio de Janeiro, suggesting that its adaptations to the high temperatures of honey bee colonies also facilitates its survival in warm-blooded mammals [32]. Other supposedly insect-restricted species are also known to occasionally cause illness in humans, including a strain of the mosquito parasite Crithidia fasciculata that was recently implicated in an outbreak of visceral leishmaniasis-like illness in Brazil [33]. My hope is that this research helps to illuminate the evolution of heat tolerance in trypanosomatids in ways that protect both beneficial insects and the warm-blooded humans and wildlife vulnerable to trypanosomatid infections.
- Schwarz RS, Bauchan GR, Murphy CA, Ravoet J, de Graaf DC, Evans JD. 2015 Characterization of Two Species of Trypanosomatidae from the Honey Bee Apis mellifera: Crithidia mellificae Langridge and McGhee, and Lotmaria passim n. gen., n. sp. J. Eukaryot. Microbiol. 62, 567–583. (doi:10.1111/jeu.12209)
- Ravoet J, Maharramov J, Meeus I, De Smet L, Wenseleers T, Smagghe G, de Graaf DC. 2013 Comprehensive bee pathogen screening in Belgium reveals Crithidia mellificae as a new contributory factor to winter mortality. PLOS ONE 8, e72443.
- vanEngelsdorp D et al. 2009 Colony Collapse Disorder: A Descriptive Study. PLOS ONE 4, e6481. (doi:10.1371/journal.pone.0006481)
- Waters TL. 2018 The distribution and population dynamics of the honey bee pathogens Crithidia mellificae and Lotmaria passim in New Zealand.
- Arismendi N, Bruna A, Zapata N, Vargas M. 2016 PCR-specific detection of recently described Lotmaria passim (Trypanosomatidae) in Chilean apiaries. J. Invertebr. Pathol. 134, 1–5. (doi:10.1016/j.jip.2015.12.008)
- Cornman RS, Tarpy DR, Chen Y, Jeffreys L, Lopez D, Pettis JS, vanEngelsdorp D, Evans JD. 2012 Pathogen Webs in Collapsing Honey Bee Colonies. PLOS ONE 7, e43562. (doi:10.1371/journal.pone.0043562)
- Morimoto T, Kojima Y, Yoshiyama M, Kimura K, Yang B, Peng G, Kadowaki T. 2013 Molecular detection of protozoan parasites infecting Apis mellifera colonies in Japan. Environ. Microbiol. Rep. 5, 74–77. (doi:https://doi.org/10.1111/j.1758-2229.2012.00385.x)
- Brown MJF, Schmid‐Hempel R, Schmid‐Hempel P. 2003 Strong context-dependent virulence in a host–parasite system: reconciling genetic evidence with theory. J. Anim. Ecol. 72, 994–1002. (doi:https://doi.org/10.1046/j.1365-2656.2003.00770.x)
- Otterstatter MC, Gegear RJ, Colla SR, Thomson JD. 2005 Effects of parasitic mites and protozoa on the flower constancy and foraging rate of bumble bees. Behav. Ecol. Sociobiol. 58, 383–389. (doi:10.1007/s00265-005-0945-3)
- Fauser A, Sandrock C, Neumann P, Sadd BM. 2017 Neonicotinoids override a parasite exposure impact on hibernation success of a key bumblebee pollinator. Ecol. Entomol. 42, 306–314. (doi:10.1111/een.12385)
- Shykoff JA, Schmid-Hempel P. 1991 Incidence and effects of four parasites in natural populations of bumble bees in Switzerland. Apidologie 22, 117–125. (doi:10.1051/apido:19910204)
- James RR. 2005 Temperature and chalkbrood development in the alfalfa leafcutting bee, Megachile rotundata. Apidologie 36, 15–23. (doi:10.1051/apido:2004065)
- Dalmon A, Peruzzi M, Le Conte Y, Alaux C, Pioz M. 2019 Temperature-driven changes in viral loads in the honey bee Apis mellifera. J. Invertebr. Pathol. 160, 87–94. (doi:10.1016/j.jip.2018.12.005)
- Martín-Hernández R, Meana A, García-Palencia P, Marín P, Botías C, Garrido-Bailón E, Barrios L, Higes M. 2009 Effect of temperature on the biotic potential of honeybee microsporidia. Appl. Environ. Microbiol. 75, 2554–2557. (doi:10.1128/AEM.02908-08)
- Zheng H, Powell JE, Steele MI, Dietrich C, Moran NA. 2017 Honeybee gut microbiota promotes host weight gain via bacterial metabolism and hormonal signaling. Proc. Natl. Acad. Sci. 114, 4775–4780. (doi:10.1073/pnas.1701819114)
- Zilberstein D, Shapira M. 1994 THE ROLE OF pH AND TEMPERATURE IN THE DEVELOPMENT OF LEISHMANIA PARASITES. Annu. Rev. Microbiol. 48, 449–470. (doi:10.1146/annurev.mi.48.100194.002313)
- Palmer-Young EC, Raffel TR, McFrederick Quinn S. 2018 Temperature-mediated inhibition of a bumblebee parasite by an intestinal symbiont. Proc. R. Soc. B Biol. Sci. 285, 20182041. (doi:10.1098/rspb.2018.2041)
- Palmer-Young EC, Raffel TR, McFrederick QS. 2019 pH-mediated inhibition of a bumble bee parasite by an intestinal symbiont. Parasitology 146, 380–388. (doi:10.1017/S0031182018001555)
- Palmer-Young EC, Ngor L, Nevarez RB, Rothman JA, Raffel TR, McFrederick QS. 2019 Temperature dependence of parasitic infection and gut bacterial communities in bumble bees. Environ. Microbiol. 21, 4706–4723. (doi:10.1111/1462-2920.14805)
- Dadd RH. 1975 Alkalinity within the midgut of mosquito larvae with alkaline-active digestive enzymes. J. Insect Physiol. 21, 1847–1853. (doi:10.1016/0022-1910(75)90252-8)
- Langridge DF. 1966 Flagellated protozoa (Trypanosomidae) in the honey bee, Apis mellifera, in Australia. J. Invertebr. Pathol. 8, 124–125. (doi:10.1016/0022-2011(66)90112-1)
- Langridge DF, McGhee RB. 1967 Crithidia mellificae n. sp. an acidophilic trypanosomatid of the honey bee Apis mellifera. J. Protozool. 14, 485–487. (doi:10.1111/j.1550-7408.1967.tb02033.x)
- Meikle WG, Weiss M, Maes PW, Fitz W, Snyder LA, Sheehan T, Mott BM, Anderson KE. 2017 Internal hive temperature as a means of monitoring honey bee colony health in a migratory beekeeping operation before and during winter. Apidologie 48, 666–680. (doi:10.1007/s13592-017-0512-8)
- Mardan M, Kevan PG. 1989 Honeybees and ‘yellow rain’. Nature 341, 191–191. (doi:10.1038/341191a0)
- Ngor L, Palmer-Young EC, Nevarez RB, Russell KA, Leger L, Giacomini SJ, Pinilla-Gallego MS, Irwin RE, McFrederick QS. 2020 Cross-infectivity of honey and bumble bee-associated parasites across three bee families. Parasitology 147, 1290–1304. (doi:10.1017/S0031182020001018)
- Jones JC, Myerscough MR, Graham S, Oldroyd BP. 2004 Honey Bee Nest Thermoregulation: Diversity Promotes Stability. Science 305, 402–404. (doi:10.1126/science.1096340)
- Graystock P, Ng WH, Parks K, Tripodi AD, Muñiz PA, Fersch AA, Myers CR, McFrederick QS, McArt SH. 2020 Dominant bee species and floral abundance drive parasite temporal dynamics in plant-pollinator communities. Nat. Ecol. Evol. 4, 1358–1367. (doi:10.1038/s41559-020-1247-x)
- Lukeš J, Skalický T, Týč J, Votýpka J, Yurchenko V. 2014 Evolution of parasitism in kinetoplastid flagellates. Mol. Biochem. Parasitol. 195, 115–122. (doi:10.1016/j.molbiopara.2014.05.007)
- Steverding D. 2017 The history of leishmaniasis. Parasit. Vectors 10, 82. (doi:10.1186/s13071-017-2028-5)
- McGwire BS, Satoskar AR. 2014 Leishmaniasis: clinical syndromes and treatment. QJM Int. J. Med. 107, 7–14. (doi:10.1093/qjmed/hct116)
- Fahrenholz L, Lamprecht I, Schricker B. 1989 Thermal investigations of a honey bee colony: thermoregulation of the hive during summer and winter and heat production of members of different bee castes. J. Comp. Physiol. B 159, 551–560. (doi:10.1007/BF00694379)
- Dario MA, Lisboa CV, Silva MV, Herrera HM, Rocha FL, Furtado MC, Moratelli R, Rodrigues Roque AL, Jansen AM. 2021 Crithidia mellificae infection in different mammalian species in Brazil. Int. J. Parasitol. Parasites Wildl. 15, 58–69. (doi:10.1016/j.ijppaw.2021.04.003)
- Maruyama SR et al. 2019 Non-Leishmania Parasite in Fatal Visceral Leishmaniasis–Like Disease, Brazil. Emerg. Infect. Dis. 25, 2088–2092. (doi:10.3201/eid2511.181548)
Photo by Michael Milverton on Unsplash
By supporting us on Patreon, you are helping us to make more videos about bees for everyone. Check it out.
Stay connected with news and updates!
Join our mailing list to receive the latest news and updates from our team.
Don't worry, your information will not be shared.
We hate SPAM. We will never sell your information, for any reason.




